Introduction
Zanubrutinib and orelabrutinib, both the next generation Bruton's tyrosine kinase inhibitors (BTKis), are used in China as treatment options for R/R MCL. A further evaluation to understand the differences between these two novel BTKis is helpful for future study. Our previously indirect comparison has suggested a favorable PFS trend in zanubrutinib over orelabrutinib in R/R MCL patients (Song Y, et al. Invest New Drugs 2023). Here, we conducted an updated analysis to indirectly compare the long-term efficacy between zanubrutinib (median follow up: 35.3 months) and orelabrutinib (median follow up: 23.8 months) in patients with R/R MCL.
Methods
Individual patient data from zanubrutinib study (BGB-3111-206, n=86; NCT03206970) were adjusted to match the patients population profile of the orelabrutinib study (ICP-CL-00102, n=106; NCT03494179). As both trials were single-arm and were not linked through a common control arm, an unanchored matching-adjusted indirect comparison (MAIC) was performed to adjust for effect modifiers and prognostic variables. The baseline characteristics included in population adjustment were identified from literature review and clinical experts' assessment, which were sex, bulky disease, bone marrow involvement, disease stage, simplified Mantle Cell Lymphoma International Prognostic Index, prior autologous stem cell transplantation, and the number of prior lines of treatment. The efficacy outcomes included investigator-assessed progression free survival (PFS), overall survival (OS), and overall response rate (ORR). After matching, a weighted Cox model or a weighted logistic model will be employed to analyze the PFS and OS through hazard ratio (HR) or to analyze the ORR through odds ratio (OR). Response evaluations were only CT-based assessment in orelabrutinib study, while PET- and CT-based assessment were both performed in zanubrutinib study. Thus, the PFS assessed by PET and CT of zanubrutinib study were used to compare with the PFS assessed by CT of orelabrutinib study, respectively.
Results
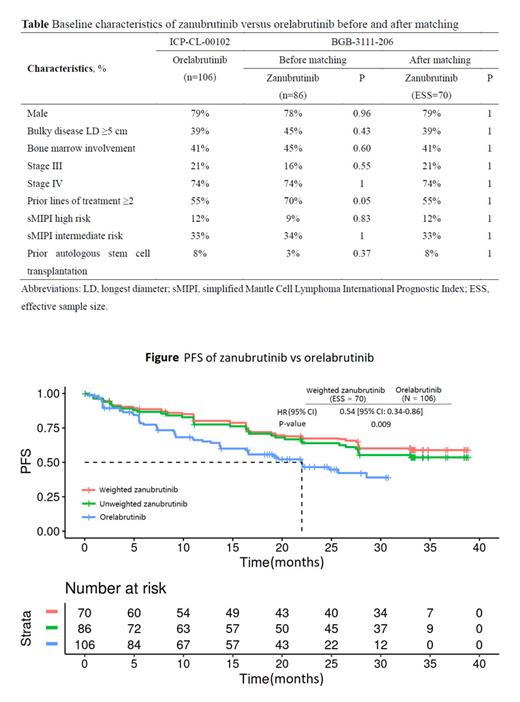
After matching, the baseline characteristics were balanced between zanubrutinib and orelabrutinib, with the (effective) sample size of 70 and 106 in zanubrutinib and orelabrutinib, respectively (Table). PFS assessed by CT was significantly longer in the zanubrutinib versus the orelabrutinib (median PFS: not estimable [NE] vs. 22.0 months; HR, 0.54 [95% CI: 0.34-0.86]; P = 0.009) (Figure). Additionally, PFS assessed by PET of zanubrutinib was also significantly longer than the PFS assessed by CT of orelabrutinib (median PFS: NE vs. 22.0 months; HR, 0.63 [95% CI: 0.40-0.99]; P = 0.044). There are clinically meaningful differences observed for both the PFS (assessed by CT or PET) of zanubrutinib versus the PFS assessed by CT of orelabrutinib and the 24-month PFS rate (assessed by CT or PET) in the zanubrutinib versus that assessed by CT in the orelabrutinib (67.3% vs. 46.5% and 62.2% vs. 46.5%, respectively). Due to very limited number of OS events, the analysis of OS was not powered enough to detect a statistical meaningful difference between two groups (median OS: NE vs. NE; HR, 0.68 [95% CI: 0.36-1.27]; P = 0.223). However, the 24-month OS rate was numerically higher in the zanubrutinib than the orelabrutinib (83.7% vs. 74.3%). ORR was comparable between zanubrutinib and orelabrutinib (85.5% vs. 82.1%; OR, 1.28 [95% CI: 0.56-2.94]; P = 0.556).
Conclusions
MAIC results demonstrated that zanubrutinib had significantly longer PFS compared with orelabrutinib in the treatment of R/R MCL patients.
Disclosures
Fang:BeiGene: Current Employment, Current equity holder in publicly-traded company. Xu:BeiGene: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal